Overview
History of liver transplantation
Starzl performed the first human liver transplant in 1963. [1] Since then, the evolution of immunosuppression and the development of new surgical approaches have led to the establishment of 100 transplant centers in the United States. Surgeons currently perform more than 500 pediatric transplantations per year.
Introduction to pediatric liver transplantation
Liver transplantation is a treatment, used in appropriately selected patients, for acute and chronic liver failure due to any cause. It is not indicated if an acceptable alternative is available or if contraindications are present (eg, some cases of malignancy, terminal conditions, poor expected quality of outcome).
Pediatric patients account for about 12.5% of liver transplant recipients. When a pediatric patient is likely to require a liver transplant, the medical management is generally divided into pretransplant and posttransplant periods, with the posttransplant period further separated into early and late time frames.
Medical treatment, surgery, and postsurgical care can be broken into 4 basic steps:
- Candidate evaluation
- Waiting period
- Surgery
- Postsurgical care
Go to Pediatric Fulminant Hepatic Failure for more complete information on this topic.
Pretransplantation care
Pretransplantation care needs to take into consideration potentially prolonged waiting periods and to project far in advance when transplantation might be required. By initiating the pretransplant workup early, one can work toward maximizing the nutritional status.
Nutritional status impacts both pretransplant and posttransplant outcomes, especially in the pediatric population, because of an increased incidence of cholestatic liver diseases. Cholestatic liver diseases lead to fat malabsorption, which causes a deficiency of calories as well as fat-soluble vitamins. [2]
Pediatric patients can greatly benefit from caloric assessments and supplemental tube feedings as indicated. Furthermore, parenteral feedings are sometimes warranted in the most nutritionally deprived patients with end-stage liver disease. The optimization of nutritional status in pediatric patients has translated into improved survival after transplantation, fewer infections, and a reduction of surgical complications. [3]
Neonatal liver transplantation
Liver transplantation has been successfully extended to neonates. [4] Acute liver failure from hemochromatosis, leading to a histologic diagnosis of giant-cell hepatitis, is the primary indication for liver transplantation in the neonatal population. Because of size discrepancies between the recipient and the donor pool, partial liver grafts are usually used for this population of patients. [5]
Although neonates appear to be more immunotolerant to transplanted organs, their immature immune systems combined with immunosuppression increases the risk for infectious complications. Among neonatal transplant recipients, vascular thrombosis is the major complication, dramatically reducing survival.
Indications for pediatric liver transplantation
About 50% of the pediatric patients who require a liver transplant have biliary atresia. Other disease states that progress to end-stage liver disease among pediatric patients and require liver transplantation include metabolic disorders and progressive intrahepatic cholestasis.
Examples of metabolic derangements include Wilson disease, alpha 1-antitrypsin deficiency, tyrosinemia, and hemochromatosis. Other metabolic disease states leading to hepatic dysfunction include the following [6] :
-
Crigler-Najjar syndrome
-
Glycogenosis
-
Hyperoxaluria
-
Metabolic respiratory chain deficiencies
-
Familial hypercholesterolemia
-
Methylmalonyl aciduria
Contraindications to pediatric liver transplantation
Transplantation is not indicated if an acceptable alternative is available or if contraindications, such as malignancy, a terminal condition, or poor expected outcome exist.
Liver anatomy
The liver is located in the right upper portion of the abdominal cavity, just beneath the diaphragm, and is protected by the rib cage. It sits on top of the stomach, right kidney, and intestines. It is supplied with blood by the portal vein, which drains the splenic, intestinal, and colonic areas and is a rich source of nutrients and substances absorbed from the gut.
It is also supplied by the hepatic artery (usually a branch of the celiac artery), which provides most of the liver's oxygenated blood. The liver consists of 2 main lobes, which are made up of thousands of lobules. These lobules are connected to small ducts that connect with larger ducts, ultimately to form the common hepatic duct. The common hepatic duct transports the bile produced by the liver cells to the gallbladder and duodenum. [7] (See the images below.)
 Anterior view of the liver. A large right and a smaller left lobe make up the liver. The gallbladder can sometimes be seen lying underneath the liver. The round ligament (ligamentum teres) of liver (obliterated umbilical vein) separates the right lobe from the left lobe of the liver. The diaphragm lies superior to the 2 lobes of the liver.
Anterior view of the liver. A large right and a smaller left lobe make up the liver. The gallbladder can sometimes be seen lying underneath the liver. The round ligament (ligamentum teres) of liver (obliterated umbilical vein) separates the right lobe from the left lobe of the liver. The diaphragm lies superior to the 2 lobes of the liver.
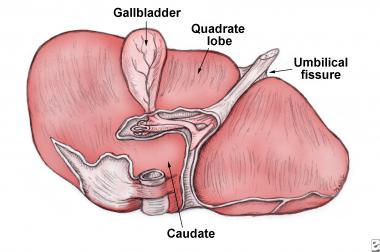 Visceral surface of liver. The portion of the right lobe located anterior to the fissure is called the quadrate lobe.
Visceral surface of liver. The portion of the right lobe located anterior to the fissure is called the quadrate lobe.
 Posterior side view of the liver. The inferior vena cava (IVC) is seen in the deep groove. It is protected on the right side by a layer of fibrous tissue. Various ligaments serve to attach the liver to the nearby anatomical regions such as the diaphragm. (RHV = right hepatic vein, LHV = left hepatic vein, MHV = middle hepatic vein).
Posterior side view of the liver. The inferior vena cava (IVC) is seen in the deep groove. It is protected on the right side by a layer of fibrous tissue. Various ligaments serve to attach the liver to the nearby anatomical regions such as the diaphragm. (RHV = right hepatic vein, LHV = left hepatic vein, MHV = middle hepatic vein).
 Liver segments according to functional division, seen in the normal anatomical position within the abdomen.
Liver segments according to functional division, seen in the normal anatomical position within the abdomen.
Candidate evaluation
Once a liver transplant is considered, a team of specially trained staff usually evaluates the patient to establish whether the patient would be a good candidate for a liver transplant. The team includes the following specialists:
-
Hepatologists (medical liver specialists)
-
Transplant surgeons
-
Social workers
-
Psychologists, psychiatrists, or both
-
Nurses
-
Transplant coordinators
When a pediatric patient is likely to require a liver transplantation, the medical management is generally divided into pretransplant and posttransplant periods, with the posttransplant follow-up further separated into early and late periods.
Waiting period
Once a pediatric patient is found to be a suitable candidate for a liver transplant, the patient's name is placed on a waiting list for an organ. Unfortunately, many more potential recipients are on the waiting list than there are organs available each year.
At most transplantation centers, the decision to be placed on a waiting list is determined by a multidisciplinary committee. Although not pediatric specific, an observational multicenter study reported that the decision process primarily involves reviewing possible reasons for patient exclusion, which may include patients who are not sick enough, too sick, or too old; other reported factors were the presence of nonhepatic comorbid conditions, substance abuse problems, or other psychosocial barriers. [8]
Need stratification
Candidates waiting to receive donor livers are stratified according to the severity of their illness and blood type. Organ allocations are generally based on medical urgency more than the length of time a person has been on the waiting list. Candidates with fulminant hepatic failure (status 1) are allocated organs ahead of all other waiting patients.
The stratification of deceased organ donation was formulated by the United Network for Organ Sharing (UNOS). [9] This system uses a risk determination based on a 3-month pretransplant assessment risk profile to assign priority and organ allocation to the most severely ill patients.
Patients who qualify for liver transplantation are assigned a score based on a nationwide ranking system. This system is called the Model for End-Stage Liver Disease (MELD) for adults and the Pediatric End-Stage Liver Disease (PELD) for patients younger than 12 years.
MELD, which was inaugurated in 2002, replaced the previous 3 medical severity staging systems for adult liver transplant candidates with chronic liver diseases. MELD uses a mathematical formula based on a patient's creatinine level, international normalized ratio (INR) for prothrombin time, and bilirubin. The MELD score quantifies the risk of death within 3 months (the higher the score, the higher the mortality).
The PELD score differs in that its calculation also includes albumin level, growth failure, and the patient's age when first placed on the waiting list, and it does not include the creatinine level. [6, 10, 11] See the MELD Score calculator and the PELD Score calculator.
The PELD score is calculated as follows (laboratory values < 1 are set to 1 for the purposes of the PELD score calculation):
PELD score = 0.480 × Loge (bilirubin mg/dL) + 1.857 × Loge (INR) - 0.687 × Loge (albumin g/dL) + 0.436 if patient is < 1 y (scores for patients < 1 y listed for liver transplantation; continue to include the value assigned for age of < 1 y until the patient is actually aged 2 y) + 0.667 if the patient has growth failure (<-2 standard deviation) × 10 (then round to the nearest whole number).
Care during the waiting period
Pretransplantation care needs to take into consideration the possibility that the patient will be facing a prolonged waiting period and to project far in advance when transplantation might be required. Unfortunately, the number of children with liver deficiencies necessitating liver transplantation far exceeds the availability through donation. The mortality rate for children on the UNOS waiting list is estimated at 17%. [10]
By initiating the pretransplant workup early, one can work toward maximizing the nutritional status, a factor impacting both the pretransplant and posttransplant outcome, especially in the pediatric population, because of an increased incidence of cholestatic liver diseases such as biliary atresia. Malabsorption of fat-derived calories and fat-soluble vitamins in the setting of chronic disease causes various metabolic and disease progression problems. [3, 11, 12]
Alternatives to liver transplantation
Alternatives to liver transplantation that are currently being researched include liver support devices, artificial organ construction, and hepatocyte transplantation.
Liver support devices
Although the development of an artificial liver is an area of active research, no such substitute is currently available. Liver support devices, including the bioartificial liver and the extracorporeal liver-assist device, are systems that perfuse blood or plasma through a hepatocyte-containing device to remove cytotoxic elements. Both have undergone phase 1 clinical evaluation but with mixed results.
The Molecular Absorbent Recirculating System (MARS), developed in Germany, removes toxic substances from the blood, acting like an artificial liver. The device transports the patient's blood through a filter, where it is mixed with a "sticky" albumin. Albumin binds many compounds (eg, bilirubin, uremic toxins) and has many nonspecific binding sites for various toxins along with heavy metals. The toxins in the blood then attach to the albumin molecules, thus removing them.
Artificial organ construction
Tissue organ construction for the liver remains experimental at this time, with the hopes that one day it will be an alternative to available treatments.
Hepatocyte transplantation
Hepatocyte transplantation is also being studied as an adjunct treatment for acute hepatic failure. Harvested human and animal hepatocytes have shown only modest success, however. The major obstacles to xenotransplantation have been the potential spread of infection from animal to human recipient and hyperacute and vascular rejection as a result of the cross-species transplant. Although hepatocyte transplantation appears to be promising, its future role will likely be in gene transfer technology, which is still in the research phase.
Preparation
Anesthesia in pediatric liver transplantation
Living donors receive general anesthesia and immediate transplantation of a portion of their liver into the recipient. [13, 14] Therefore, the patient receiving the transplant is prepared for surgery within the same time frame as the donor.
A liver from a deceased donor must be transplanted into the recipient within 12-18 hours. A team of surgeons and anesthesiologists performs an operation to remove the liver from the donor. The liver is then preserved and packed for transport. These procedures are performed using standard surgical practices and sterile techniques. Upon completion of the operation, the incisions are closed, and the donor's body is prepared for funeral or cremation. If desired, an open-casket funeral remains possible.
Technique
Overview of liver transplantation
Most liver transplants are orthotopically performed, meaning the new liver is placed in the same location as the diseased liver. This requires that the diseased liver be removed. This portion of the operation is critical and can take several hours, depending on the extent of previous surgery and the adhesions and scar tissue that developed.
If the new liver is a whole liver, the intrahepatic portion of the inferior vena cava can be removed as well, or it can be left in the recipient and the new liver "piggybacked" onto the cava. If the new liver is coming from a living donor or is the result of a reduction or splitting of the deceased donor's liver, then the inferior vena cava must be left in place and the new liver will be anastomosed to the native vena cava. The preferred preservation time for the new liver is less than 12 hours, although the maximum time is reported to be 24 hours.
The implantation requires the reestablishment of blood flow to the liver via the portal vein and hepatic artery and the reestablishment of blood flow away from the liver via the hepatic veins. After the blood flow has been restored, the bile duct's continuity with the GI tract must be established. In pediatric transplantation, this is usually via a hepaticojejunostomy.
A significant advance in transplantation has been the use of living donor and split-liver grafts. Technical challenges include vascular anatomy, sufficient volume for metabolic demands of the patient, and biliary drainage.
The use of split livers from deceased donors and partial grafts from living donors has yielded encouraging results in terms of graft viability. A careful donor and recipient selection process has been advocated to decrease the risk of primary nonfunction in split-liver grafts. The use of living donation in pediatric transplantation is well established and has been shown to be associated with excellent results in the child and is generally safe for the donor (20% morbidity, 0.01% mortality).
Post-Procedure
Immediate postoperative care
Following liver transplant surgery, patients frequently remain on a ventilator for the first 24-48 hours. Patients are moved out of the pediatric ICU (PICU) in a few days, depending on their recovery.
Reintroduction of oral intake can begin within the week following surgery. Typically, hospital stays range from 1-2 weeks. Blood tests are performed within the first few weeks following transplantation to confirm correct medication levels.
Prior to discharge, the transplant team provides follow-up care and medication instructions. The patient's and caregivers' questions are answered, and signs of rejection are discussed with the patient in an age-appropriate manner and with the family. The patient and family should be instructed to continue a rehabilitation program that includes exercise, proper nutrition, and the continuation of immunosuppression and other medications.
Generally, living donors do not have any restrictions or specific medications or special diet as a result of liver donation.
Posttransplant immunosuppression
Immunosuppression protocols focus on the use of calcineurin inhibitors, such as tacrolimus, cyclosporine (CSA), and intravenous corticosteroids (eg, methylprednisolone [Solu-Medrol]). These medications are useful because they promote downregulation of T cells.
Tacrolimus has mostly replaced CSA because studies demonstrate reduced rates of steroid-resistant acute rejection in randomized trials. The use of tacrolimus in patients receiving a liver transplant has been shown to be an independent variable with a survival advantage in recipients over a 5-year period.
Interestingly, the liver is relatively tolerogenic compared with other transplanted organs, which has allowed successful transplantation between poor HLA matches and even patients who were ABO incompatible. The results from one study noted that ABO-incompatible liver transplantation can be used safely in infants weighing less than 5 kg. No significant differences were noted in patient survival, graft survival, vascular complications, or biliary complications, and rejection rates were similar to those who received ABO-compatible liver transplants; after a median follow-up of 34 months, the 5 patients who were ABO incompatible are all alive and without graft failure. [15]
Because of the recognized CNS disturbances and nephrotoxicity associated with use of calcineurin inhibitors, clinicians are investigating other drug regimens for liver transplant recipients. One such drug is mycophenolate mofetil (MMF; CellCept), which is an integral part of immunosuppression following kidney transplant.
The activity of MMF is via noncompetitive reversible inhibition of inosine monophosphate dehydrogenase (IMPDH) in the purine biosynthesis pathway. Inhibition of IMPDH results in a depletion of guanosine triphosphate and deoxyguanosine triphosphate, thereby inhibiting T- and B-cell proliferation, cytotoxic T-cell generation, and antibody secretion.
Studies indicate an improvement in creatinine clearance with the initiation of MMF therapy and a reduction in the calcineurin inhibitor dose (as compared with calcineurin inhibition alone). Although MMF does not cause kidney toxicity, it has been implicated in gastrointestinal dysfunction and bone marrow suppression. The exact role of MMF as an immunosuppressive agent for pediatric patients has yet to be elucidated. [16]
Sirolimus has also been introduced into immunosuppressive protocols for liver transplant recipients. Sirolimus is not recommended immediately after transplantation because of a question surrounding an increased risk of hepatic artery thrombosis. A higher incidence of wound complications has been reported in patients receiving sirolimus immediately after transplantation. Later after transplantation, sirolimus may be introduced, with the goal of reducing the dosage of cyclosporine or tacrolimus to improve renal function. After a minimum follow-up of 6 months, data from one study noted that sirolimus was effective in rescuing pediatric patients with acute and chronic allograft rejection after liver transplantation; those with calcineurin inhibitor–induced nephropathy realized improved renal function. [17]
Long-term protocols are focused on sustaining graft acceptance while limiting morbidity. Transplant centers have reported cases of successful withdrawal of immunosuppression while achieving prolonged graft survival. In addition, aggressive attempts to limit steroids have demonstrated long-term beneficial effects.
Induction, maintenance, and antirejection therapy
Induction immunosuppression therapy refers to all medications given in intensified doses immediately after transplantation for the purpose of preventing acute rejection. Although the drugs may be continued after discharge for the first 30 days after transplant, they are usually not used long-term for immunosuppression maintenance.
Thymoglobulin and basiliximab have been used for induction immunosuppression in pediatrics. The medication used varies; the clinical situation determines which medications are selected.
Maintenance immunosuppression therapy includes all immunomodulation medications given before, during, or after the transplant with the intention of maintaining them long term.
Antirejection immunosuppression therapy includes all immunosuppressive medication administered for the purpose of treating an acute rejection episode during the initial posttransplant period or during a specific follow-up period, usually as long as 30 days after the diagnosis of acute rejection.
Two thirds of rejection episodes occur within 3 months of the transplant. Rejection episodes are most commonly treated with intermittent doses (ie, pulse doses) of corticosteroids. [3, 18] Other medications can include antithymocyte globulin [Thymoglobulin]) or muromonab-CD3.
Prognosis following liver transplantation
In the last 2 decades, orthotopic liver transplantation has been associated with 1-year survival rates of 80-90%. [19] (See the table below.)
Table. Graft and Patient Survival Rates for Pediatric Liver Transplantation (Open Table in a new window)
| Donor Type | Graft Survival | Patient Survival | ||||
| 6 mo, % | 1 y, % | 3 y, % | 6 mo, % | 1 y, % | 3 y, % | |
| Split-liver, left lateral segment | 72 | 68 | 64 | 79 | 78 | 75 |
| Living donor, left lateral segment | 76 | 74 | 71 | 86 | 86 | 84 |
| Whole organ | 79 | 77 | 73 | 83 | 83 | 81 |
Compared with adults, who may suffer from recurrence of their primary illness leading to hepatic dysfunction in their transplanted grafts, children largely do not experience recurrence. This fact is reflected in the overall better allograft survival rates in the pediatric population.
Complications of liver transplantation
Complications of liver transplantation include the following:
-
Hepatic artery thrombosis
-
Biliary complications
-
Infection
-
Nephrotoxicity
-
CNS toxicity
-
Osteoporosis
-
Cardiovascular disease
-
Lymphoproliferative disorders
-
Psychosocial stress
Hepatic artery thrombosis
By far the most serious complication is hepatic artery thrombosis (HAT), which can lead to graft loss if the thrombosis occurs within the first 2 weeks of the transplant. HAT often requires surgical intervention because medical strategies such as systemic anticoagulation often prove insufficient. This complication is independently related to impaired graft survival and can necessitate retransplantation. [20]
HAT has been noted to occur more frequently with anastomoses of less than 3 mm in size. Rates of HAT as high as 4-6% have been reported. Microvascular anastomosis of the hepatic artery has decreased the incidence of this devastating complication.
HAT can present as acute liver failure, worsening bilirubin, and mental status deterioration. Other patients present with biliary leaks secondary to disruption of the anastomosis from ischemia related to the arterial thrombotic event.
HAT is often diagnosed clinically, with confirmation by ultrasound duplex assessment revealing arterial thrombosis.
If the detection is combined with surgical emergency vascularization, biliary interventional radiology, biliary surgery, and/or retransplantation, there is a 20-year patient survival rate of 80%, which is identical to pediatric transplant recipients who did not experience early HAT. [21]
In contrast, portal vein thrombosis has shown to be less deleterious to organ recipients. Clinical manifestations may be limited to hypersplenism. [3]
In cases of portal vein obstruction after liver transplantation, a technique for portal vein recanalization through a combined approach of transhepatic and minilaparotomy is technically possible with good results. This combined technique is minimally invasive and is an option to avoid or delay retransplantation or surgical treatment. [22]
Biliary complications
Biliary complications are the most frequent technical complication following liver transplantation. Bile leaks and anastomotic strictures account for most notable biliary problems encountered in the postoperative period. In some series, 70% of patients with HAT have concurrent biliary complications. [20] Biliary complications may also be associated with prolonged preservation time, ischemia, or surgical technique.
Early biliary leaks are usually best handled by reoperation. Strictures (either anastomotic or intrahepatic) can frequently be managed nonoperatively with tube drainage, stent placement, or both.
Infection
Infections after liver transplantation follow a rather consistent time course. In the early postoperative period, bacterial infection predominates. After approximately 2 weeks, fungal infections are the primary concern, and then, from 6 weeks after the transplant, viral infections dominate the infectious disease concerns for the rest of the patient's life. [23, 24]
Given this time course, most patients are given prophylactic antibiotics, antifungals, and antiviral medications from the time of the transplant. The antibiotics and antifungals are usually stopped within the first days to weeks if no signs of bacterial or fungal infections are present. Antiviral medications are usually given for the first several months.
The most frequent viral infections are cytomegalovirus (CMV), Epstein-Barr virus (EBV), adenovirus, and respiratory syncytial virus (RSV). EBV is an especially important virus because increased levels of EBV in the transplant recipient can lead to posttransplant lymphoproliferative disease (PTLD). [23, 24]
Preventing viral illness secondary to immunosuppression is a major challenge. Antiviral prophylaxis with ganciclovir has been proven to be valuable against CMV infections. CMV hyperimmune globulin may also be somewhat protective against EBV. Detection is paramount in ameliorating the negative effects of EBV- and CMV-associated illness. The use of polymerase chain reaction (PCR) for this purpose has been particularly useful. [13]
Nephrotoxicity
Although calcineurin inhibitors (eg, tacrolimus and cyclosporine) are integral to post-transplant care, these drugs have a negative effect on kidney function. This phenomenon is dose-related. Histologic examination from kidney specimens of patients receiving calcineurin inhibitors shows chronic progressive interstitial fibrosis. Importantly, creatinine levels have not been shown to be a good indicator of renal function. A rise in creatinine levels occurs only after a 50% reduction of glomerular filtration rate (GFR). [11, 25, 16, 26]
CNS toxicity
The use of tacrolimus has been shown to induce seizures. This CNS toxicity has been attributed directly to elevated serum levels of tacrolimus. [11]
Osteoporosis
Osteoporosis following liver transplantation has been described in adults with increasing frequency during a 3-month period following transplant. Osteoporosis has been postulated to occur temporally with high-intensity immunosuppression. The incidence of osteoporosis in children is still ill-defined, but the long-term consequences of osteoporosis in this population are significant. [11]
Cardiovascular disease
Patients who have received a liver transplant are at potential risk for cardiovascular events. This association is believed to be due to accelerated atherosclerosis from elevated lipid levels as well as worsened hypertension following transplantation.
Lymphoproliferative disorders
Immunosuppression has led to the development of cancers, which, for some tumor types, can be 100 times more frequent among transplant recipients than in the general population. Skin cancers account for most of the newly diagnosed cases.
Another important tumor recognized as a result of immunosuppression is PTLD. PTLD is most common during the first 2 years following transplantation. This disease process has been attributed to EBV, secondary to drug regimens that inhibit immune surveillance. EBV-related PTLD plays a major role in morbidity and mortality among pediatric transplant recipients.
PTLD affects 6-20% of posttransplant patients. Treatments for PTLD are largely centered on reducing the level of immunosuppression. Novel therapies involving the use of rituximab, a monoclonal antibody against CD20, may also prove beneficial. [3, 6, 11, 27]
Psychosocial considerations
Among pediatric patients, a unique set of psychosocial risk factors may be present, leading to poor adherence to medication regimens and subsequent graft dysfunction. Risk factors for poor compliance include child abuse, single-parent households, substance abuse, and the patient dropping out of school. A formal assessment of these risk factors must be done in order to determine the potential impact on patient compliance with postsurgical treatment. [5]
Long-term monitoring
Following liver transplantation, patients require at-home rehabilitation. Recommendations vary depending on the age of the patient. In general, if the pediatric patient is able to walk, walking is recommended to restore strength and prevent lung complications.
Follow-up visits are required for check-ups. These begin soon after the patient returns home. Initially, outpatient visits may occur weekly or even more often. As time passes, the frequency of follow-up visits usually decreases.
Medications and Devices
Medication summary
Immunosuppressive medications used in liver transplantation
Cyclosporine (CSA, Sandimmune, Neoral, Gengraf) binds to cytosolic transporter protein, cyclophilin. This complex blocks calcineurin and inhibits dephosphorylation of the nuclear factor of activated T-cells (NFAT), which prevents translocation of NFAT to the nucleus, preventing transcription of interleukin 2 (IL-2). Complications include gingival hyperplasia and hirsutism.
Tacrolimus (FK506, Prograf) is an immunosuppressive agent derived from Streptomyces tsukubaensis. It inhibits the production of IL-2. Therapeutic state is usually reached in 72 hours. Absorption of tacrolimus is not related to bile resorption. Complications include nephrotoxicity, hypertension, neurotoxicity, diabetes mellitus (2% occurrence in pediatric patients), and cardiotoxicity (rare). It has largely replaced cyclosporine.
Other immunosuppressive medications are as follows:
-
Azathioprine (Imuran) is a precursor to 6-mercaptopurine (6-MP), a purine analog that acts as an antimetabolite interfering with T-cell proliferation
-
Basiliximab (Simulect) inhibits IL-2 receptors
-
Daclizumab (Zenapax) is an immunoglobulin G (IgG) monoclonal antibody that binds to IL-2 receptors, resulting in immune system downregulation (similar to basiliximab); daclizumab was withdrawn from the United States market because of diminished use and emergence of other effective therapies
-
Muromonab-CD3 (Orthoclone OKT3) is a monoclonal antibody directed at T lymphocytes
-
Mycophenolate mofetil (MMF, CellCept) is an inosine monophosphate dehydrogenase inhibitor (IMPPH) that downregulates the de novo synthesis of nucleotides, leading to impaired T- and B-cell proliferation; it is converted to the active molecule, mycophenolate sodium, in the liver
-
Mycophenolate sodium (Myfortic) is an enteric-coated mycophenolate, not different from MMF
-
Prednisone (Deltasone, Orasone) is an oral corticosteroid integral to immunosuppression regimens; it is an effective cytokine inhibitor that reduces inflammatory response
-
Sirolimus (Rapamune), like tacrolimus, binds to FKBP-12, but with a different mechanism of action
-
Thymoglobulin is a polyclonal antithymocyte globulin
-
Anterior view of the liver. A large right and a smaller left lobe make up the liver. The gallbladder can sometimes be seen lying underneath the liver. The round ligament (ligamentum teres) of liver (obliterated umbilical vein) separates the right lobe from the left lobe of the liver. The diaphragm lies superior to the 2 lobes of the liver.
-
Visceral surface of liver. The portion of the right lobe located anterior to the fissure is called the quadrate lobe.
-
Posterior side view of the liver. The inferior vena cava (IVC) is seen in the deep groove. It is protected on the right side by a layer of fibrous tissue. Various ligaments serve to attach the liver to the nearby anatomical regions such as the diaphragm. (RHV = right hepatic vein, LHV = left hepatic vein, MHV = middle hepatic vein).
-
Liver segments according to functional division, seen in the normal anatomical position within the abdomen.
-
Liver segments according to functional division, seen outside the normal anatomical position.










